Associations between seemingly unrelated entities often reveal important lessons of nature, instructive for the understanding of mutual pathogenic roots. While investigating the clinical features of T-cell large granular lymphocytic leukemia (LGL), we noted the frequent presence of plasma cell dyscrasias/monoclonal gammopathies (MGs), chiefly MGUS. There are several potential explanations for this co-occurrence: 1) similar demographics (both conditions mostly presenting in the elderly); 2) the possibility that the two precursors originate from an initial polyclonal immune response to a common target; and 3) as alternative, that monoclonal plasma cells or the Ig product may be the targets of cytotoxic T-LGL cells in a context of tumor surveillance reaction. For instance, clonal TCR may recognize the M protein idiotype or vice versacausing aberrant activations. In addition, while MGUS should not be associated with cytopenias, responses to anti-myeloma therapies have been observed in LGL with MGUS refractory to traditional immunosuppressive therapies.
We hypothesized that the study of clinical and epidemiologic features of MGs and LGL may be helpful to elucidate some of these fundamental questions. To address this, a dual approach was applied: 1) MG-forward strategy: screening of LGL in 2348 individuals with documented diagnosis of MGs and available bone marrow exam; 2) LGL-forward strategy: search of MGs in 235 LGL cases with M protein assessment.
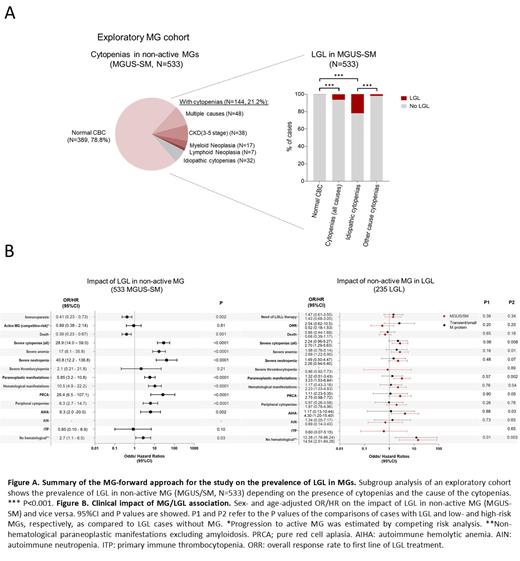
Overall, among 2348 cases with MGs, we identified 42 cases (1.8%) with concurrent evidence of LGL. We then selected an exploratory cohort of 787 MG patients with clinical annotations for further phenotypic dissection and comparative analyses. Within 533 patients with non-active MGs (i.e., MGUS or smoldering myeloma -SM), 144 (27%) had cytopenias of any type and grade, which could not be attributable to plasma cell infiltration. Screening for comorbid conditions explaining cytopenias left 32 cases (22%) presumed to be idiopathic. LGL was overrepresented in MGUS-SM with cytopenias (6 vs 0.3% in cases without counts alterations, p< .001). This frequency was higher when considering only cases with idiopathic cytopenias (22 vs 1.8% in cases with cytopenias of other causes, p< .001) ( FigA).
Reverse analysis of the LGL cohort demonstrated that 71 (30%) LGL cases had M proteins. Overall, the MG/LGL dyad was more frequently associated with concurrent autoimmune conditions (54%), as well as monoclonal B-cell lymphocytosis and other B-cell lymphoid neoplasms (19%). Next, we compared cases with MG/LGL vs those with MGs (N = 775) or LGL (N = 157) exclusively. When compared to the MGs cohort, MG/LGL cases presented a younger age (median 64 vs 70 years, p < .001), had higher frequency of M protein of the IgM type (21 vs 10%, p= .002), and oftentimes exhibited small/transient paraproteinemia (42 vs 2%, p< .001). No specific features were enriched in the MG/LGL cases when compared to the LGL cohort, except for a higher proportion of proliferations of NK-cell subtype (12 vs 4%, p= .02).
Finally, to confirm the observed association, we focused on the subgroup of non-active MGs by excluding cases with initial diagnosis of MM or lymphoma. Multivariable analysis (adjusting by sex, age and MG risk) revealed a lower risk of immunoparesis (aOR: 0.4, 95%CI: 0.2 - 0.7, p= .002) in MG/LGL as opposed to MG without LGL, whereas no effect was found in progression to MM/lymphoma (aHR: 0.9, 95%CI: 0.4 - 2.1, p= .81 in a competitive-risk setting). Moreover, when opposed to its MG and LGL counterparts, MG/LGL more frequently presented with severe cytopenias (aOR vsMGs: 28.9, 95%CI: 14.0 - 59.0, p< .001; aOR vsLGL: 2.7, 95%CI: 1.7 - 5.6, p= .008), and with immune-mediated cytopenias (aOR vsMGs: 10.5, 95%CI: 4.9 - 22.2, p< .001; aOR vsLGL: 2.8, 95%CI: 1.0 - 4.8, p= .04) ( FigB).
In conclusion, our study reveals that: 1) the association between MGUS and LGL is more than an age-related epidemiological coincidence; 2) distinctive nosologic features for the MG/LGL dyad; and 3) that even “non-active” MGs associated with LGL might be of hematological clinical significance through humoral or cellular immune mechanisms. These findings point towards alternative treatment strategies of cytopenias in MGUS and LGL (immunosuppression vs anti-myeloma therapies, respectively), and provide impetus for the investigation of tumor surveillance in MGUS vs common etiologic factors intertwining these clonopathies.
Disclosures
Maciejewski:Novartis: Honoraria, Speakers Bureau; Regeneron: Consultancy, Honoraria; Omeros: Consultancy; Alexion: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal